Chemistry Jamb Practice CBT Questions & Answers 2021
SET 1
1.A given amount of gas occupies 10.0dm5 at 4atm and 273°C. The number of moles of the gas present is [Molar volume of gas at s.t.p = 22.4dm]
A. 0.89 mol
B. 1.90 mol
C. 3.80 mol
D. 5.70 mol
Correct Answer: Option A
2.According to Charles' law, the volume of a gas becomes zero at
A. 0°C
B. -100°C
C. -273°C
D. -373°C
Correct Answer: Option C
3.A substance that is used as a ripening agent for fruits is
A. ethene
B. propane
C. methane
D. butane
Correct Answer: Option A
4. The Sulphide which is insoluble in dilute hydrochloric acid is
A. FeS
B. CuS
C. ZnS
D. Na2S
Correct Answer: Option B
5.What is the PH of 0.00 1 moldm
solution of the sodium hydroxide
A. 14
B. 13
C. 12
D. 11
Correct Answer: Option D
6. The type of bonding in [Cu(NH)]
is
A. coordinate
B. electrovalent
C. metallic
D. covalent
Correct Answer: Option A
7.Which of the following is an example of a chemical change?
A. dissolution of salt in water
B. rusting of iron
C. melting of ice
D. separating a mixture by distillation
Correct Answer: Option B
8.To what temperature must a gas at 273k be heated in order to double both its volume and pressure?
A. 298K
B. 546K
C. 819K
D. 1092K
Correct Answer: Option D
9.According to the Kinetic Theory an increase in temperature causes the kinetic energy of particles to
A. decrease
B. increase
C. be zero
D. remain constant
Correct Answer: Option B
Explanation
According to the kinetic theory of gases, when a substance is heated, the average kinetic energy of its molecules increases and this leads to a rise in temperature.
10.An element used in the production of matches is
A. nitrogen
B. aluminium
C. copper
D. sulphur
Correct Answer: Option D
11.Which of the following gases may not be dried with concentrated sulphuric acid?
A. HCl
B. NH
C. Cl
D. SO
12.The Consecutive members of an alkane homologous series differ by
A. CH
B. CH
C. CHD. CH
Correct Answer: Option B
13.A correct electrochemical series can be obtained from Na, Ca, Al, Mg, Zn, Fe, Pb, H, Cu, Hg, Ag, Au by interchanging
A. Al and Mg
B. Zn and Fe
C. Zn and Pb
D. Pb and H
Correct Answer: Option A
14.A basic postulate of the kinetic theory of gases is that the molecules of a gas move in straight lines between collisions. This implies that
A. collisions are perfectly elastic
B. forces of repulsion exist
C. forces of repulsion and attraction are in equilibrium
D. collisions are inelastic
Correct Answer: Option B
15.On which of the following is the solubility of a gaseous substance dependent?
I. Nature of solvent
II. Nature of solute
III. Temperature
IV. Pressure
A. I, II, III and IV
B. I and II only
C. II only
D. I, III and IV only
Correct Answer: Option D
16. Which of the following statements is correct about the periodic table?
A. Elements in the same period have the same number of valence electrons
B. The valence electrons of the elements in the same period increase progressively across the period
C. Elements in the same group have the same number of electron shells
D. The non-metallic Properties of the elements tend to decrease across each period
Correct Answer: Option B
Explanation
The valence electrons refer to the number of electrons in the outer shell. Across a period (horizontal row), the valence electron increases.
17. The periodic classification is an arrangement of the elements
A. atomic weights
B. isotopic weights
C. molecular weights
D. atomic numbers
Correct Answer: Option D
Explanation
Elements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the group
Elements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the group
18. If 1 litre of 2.2M sulphuric acid is poured into a bucket containing 10 litres of water and the resulting solution mixed thoroughly, the resulting sulphuric acid concentration will be
A. 2.2M
B. 1.1M
C. 0.22M
D. 0.11M
Correct Answer: Option C
19
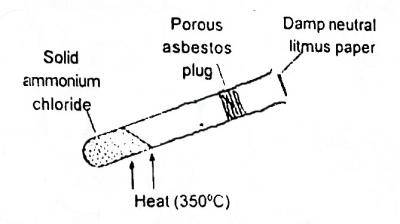
In the shown experiment (Fig. 1) the litmus paper will initially
A. be bleached
B. turn green
C. turn red
D. turn blue
Correct Answer: Option D
20. The boiling of fat and aqueous caustic soda is referred to as
A. hydrolysis
B. esterification
C. acidification
D. saponification
Correct Answer: Option D
21. Which of the following pairs of substances will react further with oxygen to form a higher oxide?
A. CO and HO
B. NO and HO
C. CO and CO
D. SO and NO
Correct Answer: Option D
22. In the preparation of oxygen by heating KCIO, in the presence of MnO
only moderate heat is needed because the catalyst acts by
A. lowering the pressure of the reaction
B. increasing the surface area of the reaction
C. increasing the rate of the reaction
D. lowering the energy barrier of the reaction
Correct Answer: Option D
23.Methanoic acid mixes with water in all proportions and has about the same boiling point as water. Which of the following methods would you adopt to obtain pure water from a mixture of Sand, water and methanoic acid?
A. Fractional distillation
B. Filtration followed by distillation
C. Neutralization with sodium hydroxide followed by distillation
D. Neutralization with sodium hydroxide followed by filtration
Correct Answer: Option B
Explanation
the first process would be FILTRATION in order to remove the sand, then DISTILLATION is used to separate the liduid.
24. A quantity of electricity liberates 3.6g of Silver from its salt. What mass of aluminium Will be liberated from its salt by the same quantity of electricity? [Al = 27, Ag = 108].
A. 2.7g
B. 1.2g
C. 0.9g
D. 0.3g
Correct Answer: Option A
25.Suitable reagents for the laboratory preparation nitrogen are
A. sodium dioxonitrate(III) and ammonium chloride
B. sodium trioxonitrate(V) and ammonium chloride
C. sodium chloride and ammonium trioxonitrate(V)
D. sodium chloride and ammonium di-ozonitrate(III)
Correct Answer: Option A
26. The number of electrons in the valence shell of an element of atomic number 14 is?
A. 1
B. 2
C. 3
D. 4
Correct Answer: Option D








Helpful
ReplyDeleteIt good you check this
ReplyDelete