
Click Here For Chemistry 2021 JAMB Questions EXPO
1.A given amount of gas occupies 10.0dm5 at 4atm and 273°C. The number of moles of the gas present is [Molar volume of gas at s.t.p = 22.4dm]
A. 0.89 mol
B. 1.90 mol
C. 3.80 mol
D. 5.70 mol Correct Answer: Option A
2.According to Charles' law, the volume of a gas becomes zero at
A. 0°C
B. -100°C
C. -273°C
D. -373°C Correct Answer: Option C
3.A substance that is used as a ripening agent for fruits is
A. ethene
B. propane
C. methane
D. butane Correct Answer: Option A
4. The Sulphide which is insoluble in dilute hydrochloric acid is
A. FeS
B. CuS
C. ZnS
D. Na2S Correct Answer: Option B
5.What is the PH of 0.00 1 moldm
solution of the sodium hydroxide
A. 14
B. 13
C. 12
D. 11 Correct Answer: Option D
6. The type of bonding in [Cu(NH)]
is
A. coordinate
B. electrovalent
C. metallic
D. covalent Correct Answer: Option A
7.Which of the following is an example of a chemical change?
A. dissolution of salt in water
B. rusting of iron
C. melting of ice
D. separating a mixture by distillation Correct Answer: Option B
8.To what temperature must a gas at 273k be heated in order to double both its volume and pressure?
A. 298K
B. 546K
C. 819K
D. 1092K Correct Answer: Option D
9.According to the Kinetic Theory an increase in temperature causes the kinetic energy of particles to
A. decrease
B. increase
C. be zero
D. remain constant
Correct Answer: Option B
Explanation According to the kinetic theory of gases, when a substance is heated, the average kinetic energy of its molecules increases and this leads to a rise in temperature.
10.An element used in the production of matches is
A. nitrogen
B. aluminium
C. copper
D. sulphur Correct Answer: Option D
11.Which of the following gases may not be dried with concentrated sulphuric acid?
A. HCl
B. NH
C. Cl
D. SO
12.The Consecutive members of an alkane homologous series differ by
A. CH
B. CH
C. CH D. CH
Correct Answer: Option B
13.A correct electrochemical series can be obtained from Na, Ca, Al, Mg, Zn, Fe, Pb, H, Cu, Hg, Ag, Au by interchanging
A. Al and Mg
B. Zn and Fe
C. Zn and Pb
D. Pb and H Correct Answer: Option A
14.A basic postulate of the kinetic theory of gases is that the molecules of a gas move in straight lines between collisions. This implies that
A. collisions are perfectly elastic
B. forces of repulsion exist
C. forces of repulsion and attraction are in equilibrium
D. collisions are inelastic Correct Answer: Option B
15.On which of the following is the solubility of a gaseous substance dependent?
I. Nature of solvent
II. Nature of solute
III. Temperature
IV. Pressure
A. I, II, III and IV
B. I and II only
C. II only
D. I, III and IV only Correct Answer: Option D
16. Which of the following statements is correct about the periodic table?
A. Elements in the same period have the same number of valence electrons
B. The valence electrons of the elements in the same period increase progressively across the period
C. Elements in the same group have the same number of electron shells
D. The non-metallic Properties of the elements tend to decrease across each period
Correct Answer: Option B
Explanation The valence electrons refer to the number of electrons in the outer shell. Across a period (horizontal row), the valence electron increases.
17. The periodic classification is an arrangement of the elements
A. atomic weights
B. isotopic weights
C. molecular weights
D. atomic numbers
Correct Answer: Option D
Explanation Elements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the group
Elements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the group
18. If 1 litre of 2.2M sulphuric acid is poured into a bucket containing 10 litres of water and the resulting solution mixed thoroughly, the resulting sulphuric acid concentration will be
A. 2.2M
B. 1.1M
C. 0.22M
D. 0.11M
Correct Answer: Option C
19
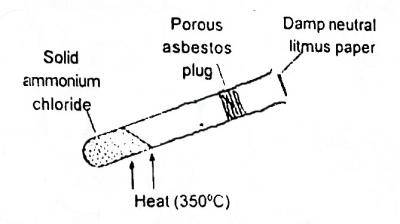
In the shown experiment (Fig. 1) the litmus paper will initially
A. be bleached
B. turn green
C. turn red
D. turn blue
Correct Answer: Option D
20. The boiling of fat and aqueous caustic soda is referred to as
A. hydrolysis
B. esterification
C. acidification
D. saponification
Correct Answer: Option D
21. Which of the following pairs of substances will react further with oxygen to form a higher oxide?
A. CO and HO
B. NO and HO
C. CO and CO
D. SO and NO
Correct Answer: Option D
22. In the preparation of oxygen by heating KCIO, in the presence of MnO only moderate heat is needed because the catalyst acts by
A. lowering the pressure of the reaction
B. increasing the surface area of the reaction
C. increasing the rate of the reaction
D. lowering the energy barrier of the reaction Correct Answer: Option D
23.Methanoic acid mixes with water in all proportions and has about the same boiling point as water. Which of the following methods would you adopt to obtain pure water from a mixture of Sand, water and methanoic acid?
A. Fractional distillation
B. Filtration followed by distillation
C. Neutralization with sodium hydroxide followed by distillation
D. Neutralization with sodium hydroxide followed by filtration
Correct Answer: Option B
Explanation the first process would be FILTRATION in order to remove the sand, then DISTILLATION is used to separate the liduid.
24. A quantity of electricity liberates 3.6g of Silver from its salt. What mass of aluminium Will be liberated from its salt by the same quantity of electricity? [Al = 27, Ag = 108].
A. 2.7g
B. 1.2g
C. 0.9g
D. 0.3g Correct Answer: Option A
25.Suitable reagents for the laboratory preparation nitrogen are
A. sodium dioxonitrate(III) and ammonium chloride
B. sodium trioxonitrate(V) and ammonium chloride
C. sodium chloride and ammonium trioxonitrate(V)
D. sodium chloride and ammonium di-ozonitrate(III)
Correct Answer: Option A 26. The number of electrons in the valence shell of an element of atomic number 14 is?
A. 1
B. 2
C. 3
D. 4
Correct Answer: Option D
27. What volume of oxygen will remain after reacting 8cm of hydrogen gas with 20cm of oxygen gas
A. 10cm
B. 12cm
C. 14cm
D. 16cm
Correct Answer: Option D
28. If one of the following oxides is heated with hydrogen or carbon using a bunsen burner. it is not reduced to the metal, Which one is it?
A. lead oxide
B. Magnesium oxide
C. Copper oxide
D. Tin oxide
Correct Answer: Option B
Explanation The oxides of Potassium, Sodium, Calcium, and Magnesium are not reduced when they react with carbon and hydrogen.
29
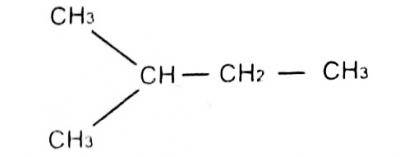
The lUPAC name for
A. 1-methyl pentane
B. 3-methylbutane
C. 2-methylbutane
D. 1-dimethyl propane Correct Answer: Option C
30. An aqueous solution of a metal salt, M. gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia Therefore the cation in M is
A. Zn
B. Ca
C. Al
D. Po
Correct Answer: Option A
31. What is the concentration of a solution containing 2g of NaOH in 100cm3 of solution? [Na = 23, O =16, H = 1]
A. 0.40 moldm
B. 0.50 moldm
C. 0.05 moldm
D. 0.30 moldm
Correct Answer: Option B
32. How many atoms are present in 6.0g of magnesium? [Mg = 24, N.A = 6.02 x 10 mol]
A. 1.20 x 10
B. 2.4 x 10
C. 1.51 x 10
D. 3.02 x 10
Correct Answer: Option C
33. The radio isotope used in industrial radiography for the rapid checking of faults in welds
and casting is?
A. carbon-14
B. phosphorus-32
C. cobalt
D. iodine-131 Correct Answer: Option C
34. Beryllium and Aluminium have similar properties because they
A. are both metals
B. belong to the same group
C. belong to the same period
D. are positioned diagonally to each other
Correct Option D
35. mE + nF -----> pG + qH
In the equation shown, the equilibrium constant is given by?
A.
B.
C.
D. Correct Answer: Option C
36
(I). 3CuO(s) + 2NH3(g) -----> 3Cu(s) + 3H2O(l) + N2(g)
(II). 2NH3(g) + 3Cl2(g) -----> 6HCl(g) + N2(g)
(III). 4NH3(g) + 3O2(g) -----> 6H2O(l) + N2(g)
The reactions represented by the equations above demonstrate the
A. basic properties of ammonia
B. acidic properties of ammonia
C. reducing properties of ammonia
D. oxidizing properties of ammonia Correct Answer: Option C
37. The salt that reacts with dilute hydrochloric acid to produce a pungent smelling gas which decolourizes acidified purple potassium tetraoxomanganate (VII) solution is
A. NaSO
B. NaSO
C. NaS
D. NaCO
Correct Answer: Option B
38.The refreshing and characteristic taste of soda water and other soft drinks is as a result of the presence of
A. carbon(IV)oxide
B. carbon(ll)oxide
C. soda
D. glucose Correct Answer: Option A
39.Which of the following are mixtures?
I. Petroleum
II. Rubber latex
III. Vulcanizer's solution
IV. Carbon sulphide
A. I, II and III
B. I, II and IV
C. I and II only
D. I and IV Correct Answer: Option A
40.A balanced chemical equation obeys the law of
A. conservation of mass
B. definite proportions
C. multiple proportions
D. conservation of energy
Correct Answer: Option A
41. When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are;
A. nitrogen and carbondioxide
B. the rare gases
C. nitrogen and oxygen
D. nitrogen and the rare gases
Correct Answer: Option D
Explanation The quick-lime is a drying agent that can dry most gases except ammonia. Hence it removes the water vapour. Quick-lime which is calcium oxide reacts with the carbon dioxide forming calcium carbonate. Hence carbon dioxide and monoxide is eliminated leaving nitrogen, and the rare gases
42 In the laboratory preparation of oxygen, the gas cannot be collected by displacement of air because
A. the density of oxygen is greater than that of air
B. the density of air is nearly the same as that of oxygen
C. oxygen is a component of air
D. air is an impure substance
Correct Answer: Option A
Explanation Oxygen is collected by the downward displacement of water because it is only slightly soluble in water and is less dense than water. It cannot be collected by downward displacement of air since oxygen will get mixed with other gases in air. Hence, the density of air is less than that of oxygen.
It cannot be collected by downward displacement of air since oxygen will get mixed with other gases in air.
43. 2KClO3(g) MNO3→ 2KCl(s) + 3O2(g)
The importance of the catalyst in the reaction above is that
A. heating may not be required before the reaction takes place
B. the reaction is controllable even at a high temperature
C. the reaction produces large quantity of oxygen
D. the reaction takes place more rapidly at a lower temperature
Correct Answer: Option D
Explanation A catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions (as at a lower temperature) than otherwise possible.
44. Which of the following is used to power steam engines?
A. lubricating oil
B. coal
C. bitumen
D. diesel oil
Correct Answer: Option B
Explanation When we burn coal on a fire, the bonds break apart and the energy is released in the form of heat. Coal contains about half as much energy per kilogram as cleaner fossil fuels such as gasoline, diesel, and kerosene—and that's one reason why steam engines have to burn so much of it.
Coal is the fuel used to power steam engines.
45. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid?
A. 5cm
B. 10cm
C. 20cm
D. 50cm
Correct Answer: Option D
Explanation 2NaOH + H
SO↔NaSO + 2HO V x 0.5M = 2(10 x 1.25)
0.5V = 2 x 12.5
0.5V = 25
V = 250.5 = 50cm
Correct computation as it follows the equation specified
46. To what volume must 300cm
of 0.60M sodium hydroxide solution be diluted to give a 0.40M solution?
A. 450cm
B. 300cm
C. 200cm
D. 150cm
Correct Answer: Option A
Explanation From the dilution law
C
V = CV C
= Initial Concentration of NaOH = 0.6M
V
= Initial Volume of NaOH = 300cm3
C
= Final Concentration of NaOH = 0.4M
V
= Final Volume of NaOH = ?
V
= V
= V
= 450 cm Correct Computation as it follows the equation specified
47. Diamond is a bad conductor of electricity because its bonding electrons are used in
A. crystal lattice formation
B. covalent bond formation
C. metallic bond formation
D. coordinate bond formation
Correct Answer: Option B
Explanation Diamond is a covalent macromolecule, and typical of a covalent substance is an insulator. All valence electrons are localized in well-defined covalent bonds and hence are not free to move through the structure
48. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as;
A. saponification
B. dehydration
C. esterification
D. hydrolysis
Correct Answer: Option C
Explanation Esterification is a chemical reaction that forms at least one ester (= a type of compound produced by reaction between acids and alcohols). Esters are produced when acids are heated with alcohols in a process called esterification. An ester can be made by an esterification reaction of a carboxylic acid and an alcohol.
49. The constituent common to duralumin and alnico is
A. Co
B. Mn
C. Al
D. Mg
Correct Answer: Option C
Explanation Constituents of duralumin are: Al, Cu, Mg, Mn.
Constituents of Alnico are: Al, Ni and Co
In 1909, the alloy of duralumin was discovered by Alfred Wilon consisting of 94% Al, 4% Cu, 1% Mg and 1% Mn(Manganese)
Alnico is an acronym referring to a family of iron alloys which in addition to iron are composed primarily of Al, Ni and Co.
50. The solubility of the solids that dissolves in a given solvent with the liberation of heat will
A. increase with an increase in temperature
B. decrease with an increase in temperature
C. decrease with a decrease in temperature
D. not be affected by changes in temperature
Correct Answer: Option A
Explanation For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperature allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular







No comments: